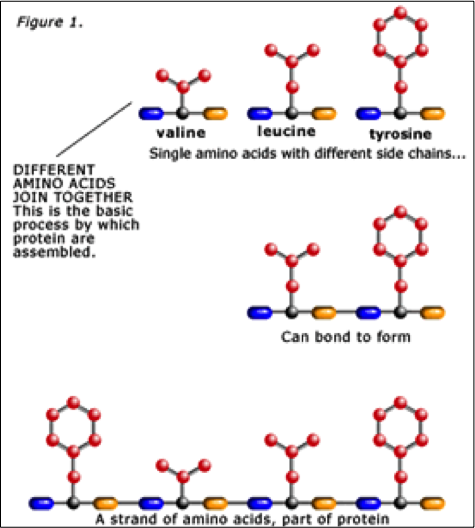
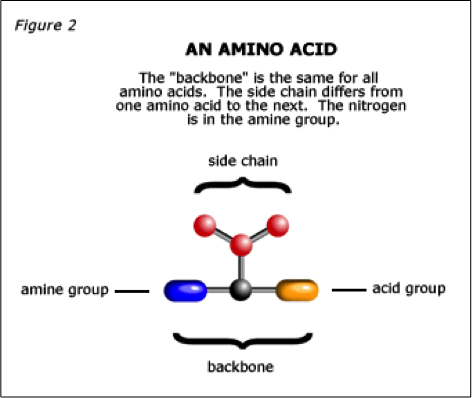
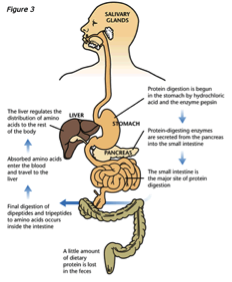
No query specified.
-
Products
-

Premium speciality range for competing at your best
More info »
High-quality support for everyday fitness, nutrition and active lifestyles
More info »
Drinks and bars for nutritious snacking and energy support on-the-go
More info »
High-protein nutrition and weight management support, with active women in mind
More info »ICE WPI RIPPED 100% WHEY PLUS MASS BCAA MAX 100%WHEY Replace Gel Plant Pro Lean Gain Slim Shake Replace Electrolyte Pre-Workout Carb Less Crunch Sculpt Protein Sculpt Shaker Horleys Shaker Horleys Drink Bottle Horleys T-Shirt
The fastest, purest protein powder available. 90% protein.
More info »
Lean Machine: advanced high protein blend, fat burning formula. 83% protein plus fat-loss aiding stimulants
More info »
Fast digesting proteins for quick delivery of amino acids for fast muscle recovery
More info »
Supercharged power, strength and size: specialised mass-gainer that's loaded with nutrients.
More info »
BCAA Complex + Electrolytes
More info »
Lean Body Nutrition: quality high-protein, low-carb support for active bodies and lifestyles. 75% Protein.
More info »
Energy On-the-Go: specialised carbohydrate replenishment supplement to support endurance sports.
More info »
Vegan Complete Protein: source of all 9 essential amino acids from pea and rice protein for nutritional support and enhanced satiety.
More info »
Muscle Food: readily absorbable complete nutrition, with low GI carbs and protein for muscle repair and growth.
More info »
No Sacrifice: filling, high protein complete meal replacement.
More info »
Energy On-the-Go: specialised carbohydrate replenishment supplement to support endurance sports.
More info »
Explosive Formula For Energy, Performance & Focus
More info »
Smart snacking with crunch: low carb protein bars.
More info »
Nutrition for Fit Women: high protein nutritional support with extra ingredients for fat-metabolism and to boost energy levels.
More info »
Sculpt Shaker 450ml
More info »
Make your protein shakes with ease.
More info »
Great for at the gym and on-the-go.
More info »
You can never have too much workout gear.
More info »
-
-
Goals
-
Lean Muscle Gain Weight / Fat Loss Muscle Mass Gain Weight Gain Pre-Workout Post-workout Recovery Energy Hydration

Surefire support for building lean muscle: products & tactics to achieve your goals faster.
More info »
Tips on nutrition, activity and motivation to help you reach your weight loss goal.
More info »
All the resources you need to support your training for maximum gains.
More info »
Achieve weight-gains for graded sport, enhanced physique and health.
More info »
Make the most of every workout with the energy and nutrients you need.
More info »
Whatever your reason for training, recovery plays a crucial role in achieving your goals.
More info »
Supply energy to working muscles, boost energy levels and feel ready to tackle your training.
More info »
Hydration for sport and fitness: faster recovery, better performance.
More info »100% WHEY PLUS BCAA MAX Sculpt Protein ICE WPI 100%WHEY BCAA MAX Slim Shake Sculpt Protein ICE WPI 100%WHEY Carb Less Crunch RIPPED MASS ICE WPI MASS Replace Gel Pre-Workout 100% WHEY PLUS BCAA MAX ICE WPI 100%WHEY Replace Gel 100%WHEY BCAA MAX Replace Gel
Fast digesting proteins for quick delivery of amino acids for fast muscle recovery
More info »
BCAA Complex + Electrolytes
More info »
Nutrition for Fit Women: high protein nutritional support with extra ingredients for fat-metabolism and to boost energy levels.
More info »
The fastest, purest protein powder available. 90% protein.
More info »
Lean Body Nutrition: quality high-protein, low-carb support for active bodies and lifestyles. 75% Protein.
More info »
BCAA Complex + Electrolytes
More info »
No Sacrifice: filling, high protein complete meal replacement.
More info »
Nutrition for Fit Women: high protein nutritional support with extra ingredients for fat-metabolism and to boost energy levels.
More info »
The fastest, purest protein powder available. 90% protein.
More info »
Lean Body Nutrition: quality high-protein, low-carb support for active bodies and lifestyles. 75% Protein.
More info »
Smart snacking with crunch: low carb protein bars.
More info »
Lean Machine: advanced high protein blend, fat burning formula. 83% protein plus fat-loss aiding stimulants
More info »
Supercharged power, strength and size: specialised mass-gainer that's loaded with nutrients.
More info »
The fastest, purest protein powder available. 90% protein.
More info »
Supercharged power, strength and size: specialised mass-gainer that's loaded with nutrients.
More info »
Energy On-the-Go: specialised carbohydrate replenishment supplement to support endurance sports.
More info »
Explosive Formula For Energy, Performance & Focus
More info »
Fast digesting proteins for quick delivery of amino acids for fast muscle recovery
More info »
BCAA Complex + Electrolytes
More info »
The fastest, purest protein powder available. 90% protein.
More info »
Lean Body Nutrition: quality high-protein, low-carb support for active bodies and lifestyles. 75% Protein.
More info »
Energy On-the-Go: specialised carbohydrate replenishment supplement to support endurance sports.
More info »
Lean Body Nutrition: quality high-protein, low-carb support for active bodies and lifestyles. 75% Protein.
More info »
BCAA Complex + Electrolytes
More info »
Energy On-the-Go: specialised carbohydrate replenishment supplement to support endurance sports.
More info »
-
- Retailers
- Latest
- About